Polycythemia Vera – Diagnostic Criteria
Prev
1 / 0 Next
Prev
1 / 0 Next
The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia:
- Polycythemia vera (PV) criteria were revised from the 2008 WHO criteria because “it is possibly underdiagnosed using the hemoglobin levels published in the fourth edition (2008 version), and the utility of BM morphology as a reproducible criterion for the diagnosis of PV is recognized”.
- The diagnostic criteria for PV have been refined to differentiate masked PV from ET (recognizing the utility of bone marrow biopsy in patients with hemoglobin levels <18.5 g/dL in men and <16.5 g/dL in women).
- Thus, the major diagnostic criteria for PV have been refined to include hemoglobin levels (>16.5 g/dL in men and >16.0 g/dL in women) or hematocrit >49% in men and >48% in women and a bone marrow biopsy to confirm the age-matched hypercellularity.
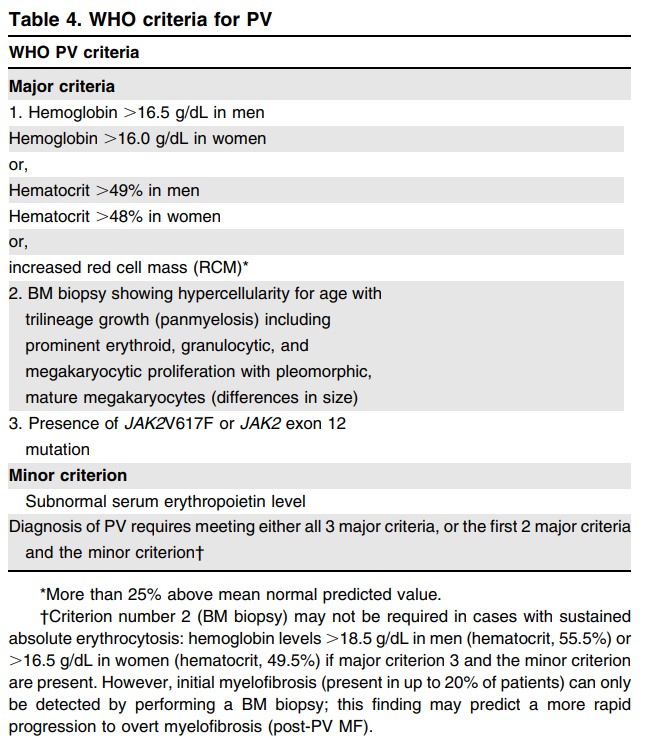
The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia:
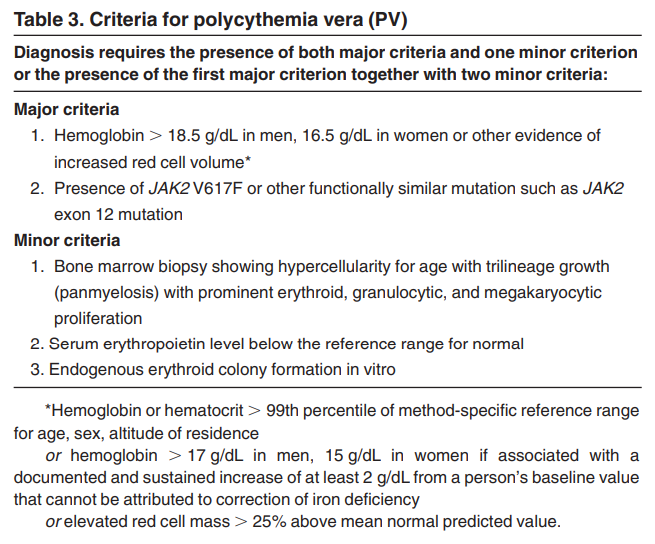
Prev
1 / 0 Next
