Chemical reactions are rearrangements of atoms as the bond between them are broken and new ones are formed. For example, in the following reaction, the C-Br bond is broken, and the C-Cl bond is formed:
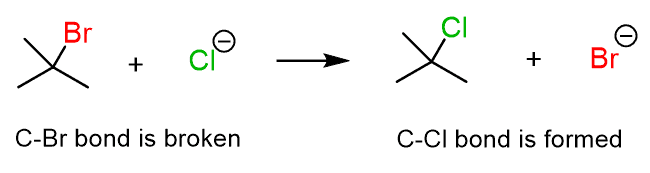
Let’s now compare this process to what is happening in the reaction between ethane and chlorine:
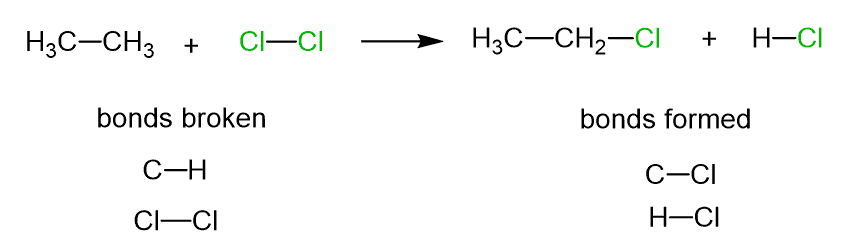
Here, the C-H bond is broken, and the C-Cl bond is formed. Now, what is the difference between these two reactions? They both involve regrouping some of the atoms. However, the mechanisms of these reactions are different.
The first one is an ionic reaction because when the bond is broken (C-Br), one atom (Br) takes both electrons of the covalent bond and the new bond is formed with two electrons coming from oxygen. This is a heterolytic cleavage also referred to as heterolysis.
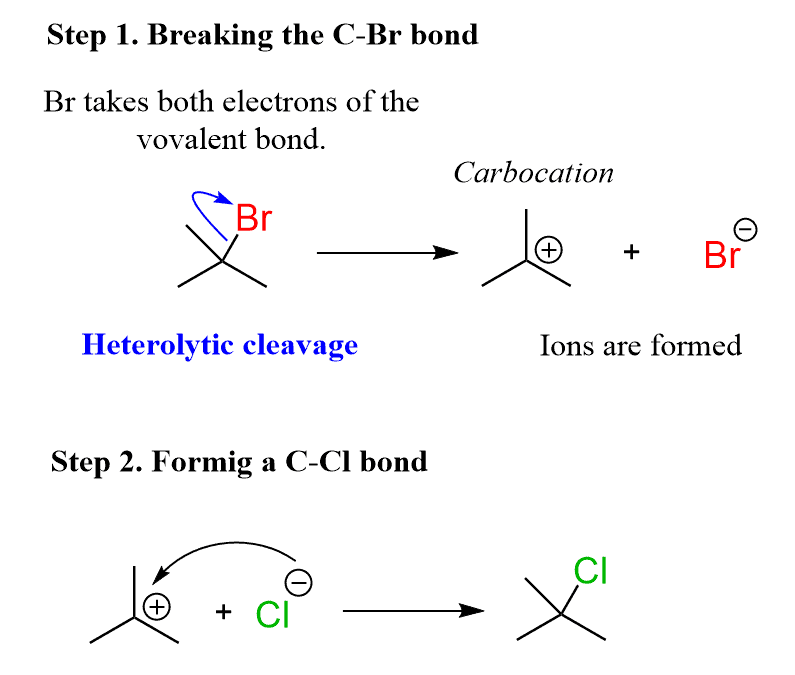
This is an SN1 reaction – a type of a nucleophilic substitution reaction which involves two or more steps. The intermediate here is a carbocation which is then attacked by the chloride ion (nucleophilic attack).
To show the mechanism (electron flow) of a heterolytic bond cleavage, full-headed arrows are used. The arrow starts from the middle of the bonds and stops at one of the atoms (usually the more electronegative atom). You can read more about curved arrows in here.
The second reaction, proceeds by a radical mechanism. The Cl-Cl bond is broken such that each Cl atom takes one electron, and this is called a homolytic cleavage:

The homolytic cleavage is shown with a half-headed arrow (fishhooks). Here, two fishhook arrows are used to show how the bond is broken. One arrow starts from the middle of the bond moving to the first atom, and the other starts from the middle of the bond and moves to the second atoms. Thus, each atom gets one electron and radical species are formed.
Radicals are reactive intermediates with a single unpaired electron, and they react very quickly to form stable molecules.
For example, the Cl radical formed in the first step quickly reacts with ethane abstraction a hydrogen and generating new radical:
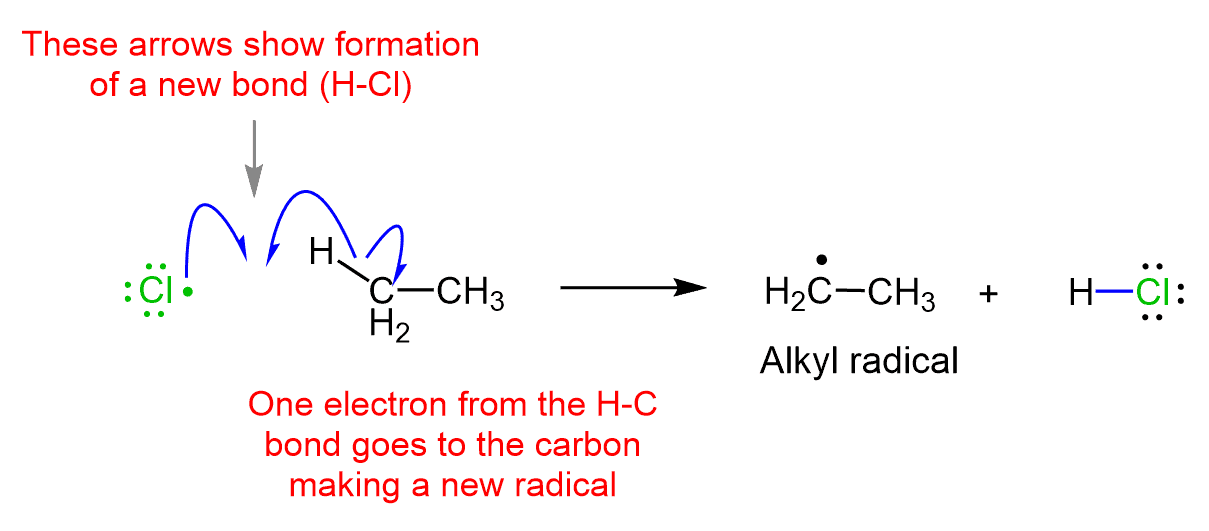
The radical is eventually trapped/quenched by another radical and a neutral molecule is formed.
The Energy of Homolytic Bond Cleavage
Both homolytic and heterolytic cleavages require energy. These are called heat of reaction or enthalpy of the reaction. Remember, enthalpy is the heat under standard pressure. The enthalpy of a homolytic cleavage is described by bond dissociation energies. These are always positive numbers since homolysis is an endothermic process. Read this post about energy changes in chemical reactions for an introduction and more details about the relation between the exothermic and endothermic processes and the signs of enthalpy change.
Bond formation, on the other hand, is an exothermic process as it always releases energy.
For example, the hydrogen molecule (H2) is formed when two free atoms of hydrogen come to an optimal proximity.
![]()
This process is associated with a 436 kJ mol−1 potential energy loss in form heat. The same amount of energy will be needed to break the bond and create two hydrogen atoms (homolytic cleavage).
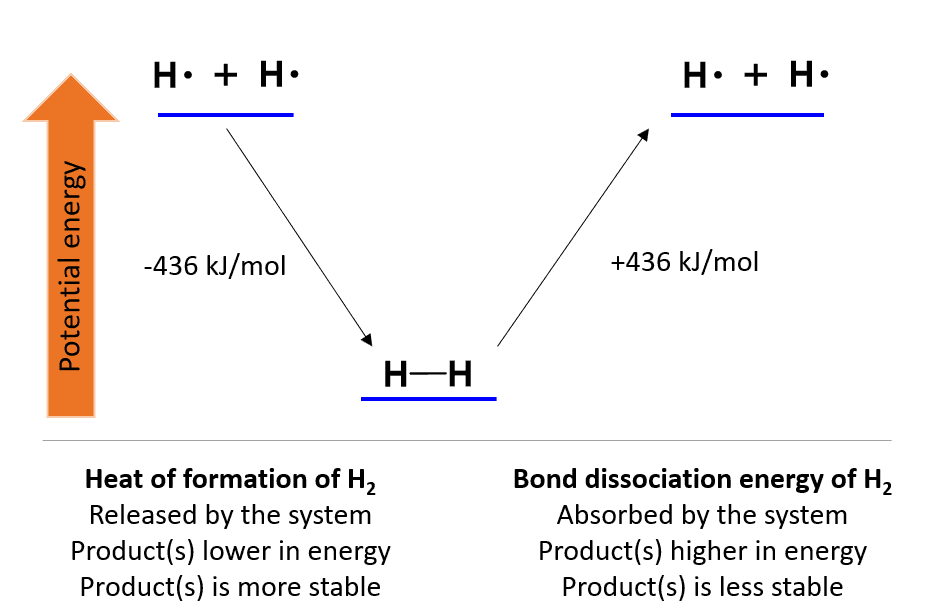
Therefore, the 436 kJ/mol is the H-H bond strength and the energy needed to break it is called the bond dissociation energy. Contrary, for the reverse process, when H2 is formed, we are talking about the heat of formation, and these two differ only with their signs.
Just like the H-H bond, the bonds between all the elements are characterized with a specific bond dissociation energy (bond strength).
The following table summarizes the bond dissociation energies of the most common bonds you will need in an organic chemistry course:

What are the bond dissociation energies used for?
We have learned the traits of bond strengths in the post about the correlation of bond length and bond strength. What we learned is that the shorter the bond the stronger it is:

As the atoms become larger, the bonds get longer and weaker as well. Longer bonds are a result of larger orbitals which presume a smaller electron density and a poor percent overlap with the s orbital of the hydrogen. Knowing this we can say that the H-F bond is stronger than the H-Cl bond because F is in the second row of the predict table and is smaller than Cl.
This is a qualitative description of the bond strength; however, the numeric data is provided in the bond dissociation energy table.
An important application of the bond dissociation energies is the calculation of the total enthalpy change in chemical reactions. So, when two molecules are reacting, these values can be used to determine the overall change of the enthalpy resulting from the unequal exo- and endo-thermic processes.
For example, the following reaction between chlorine and 2-methylpropane is an exothermic reaction ΔH° = −138 kJ/mol.
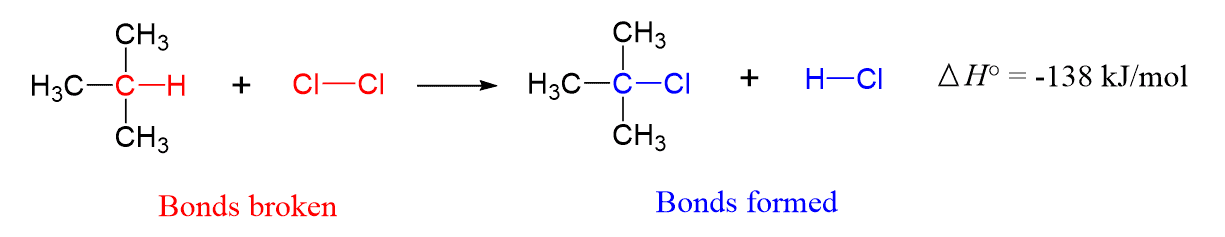
This value can be calculated form the bond dissociation energies of the breaking and forming bonds. The detailed step-by-step guide for this process will be covered in the next article.
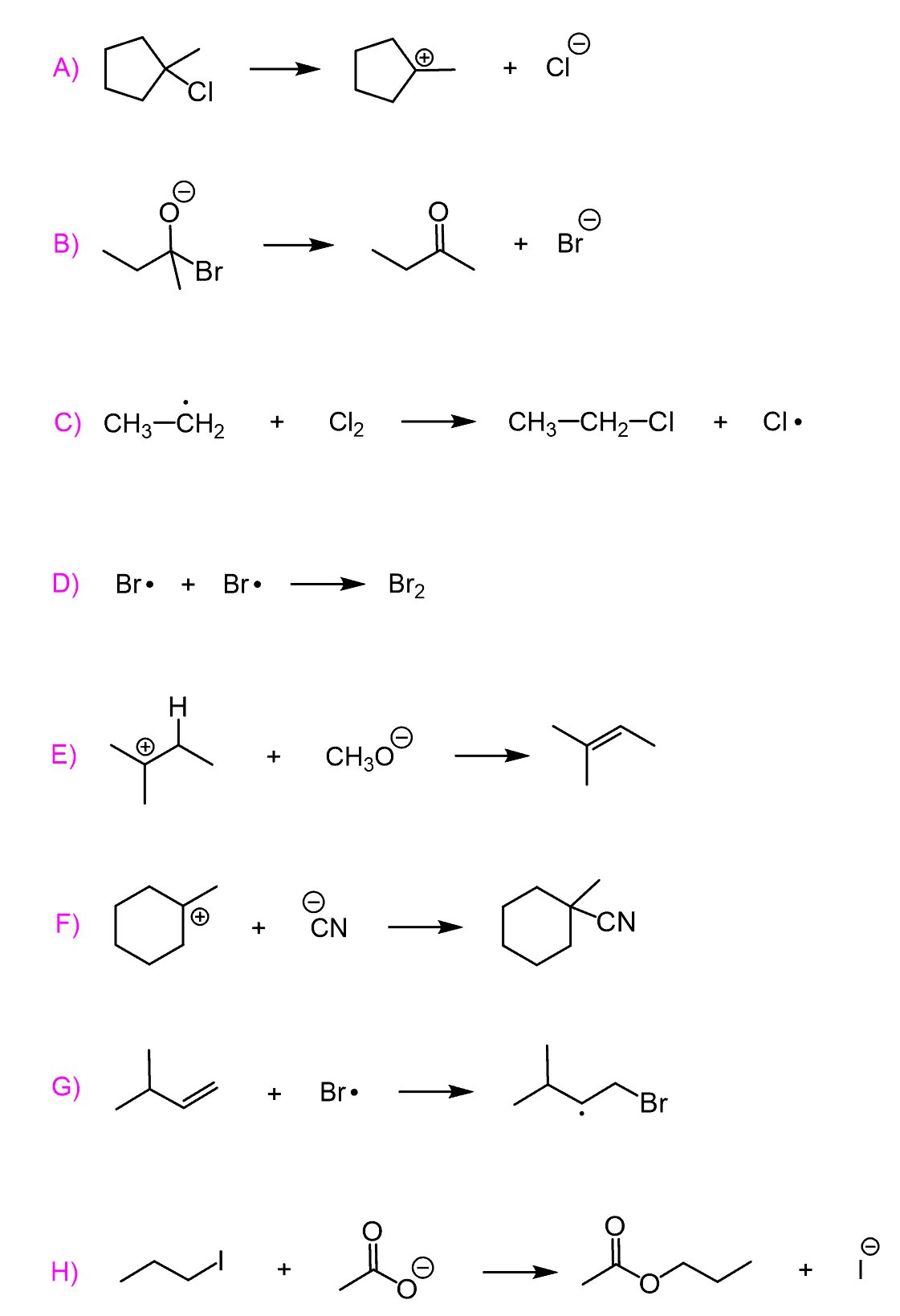
Practice
Use curved arrows to show the mechanism of each reaction.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
