Abstract
Glasses belonging to the xWO3-(30-x) Bi2O3–15Li2O–15CaO–40B2O3 (0 ≤ x ≤ 10) have been obtained by melt-quenching technique. With increasing the concentration of WO3, an increase in the measured glass transition temperature \({T}_{\mathrm{g}}\) and crystallization temperature \({T}_{\mathrm{c}}\) are observed, while the values of the thermal expansion coefficient decrease. Due to the unpaired pentavalent ions, the electron spin resonance spectra of glasses doped with \({\mathrm{WO}}_{3}\) them show unique signals. The results of Raman spectra show that the glass network is mainly constituted by [\({\mathrm{BiO}}_{6}\)], [\({\mathrm{BO}}_{3}\)], [\({\mathrm{BO}}_{4}\)], and [\({\mathrm{WO}}_{4}\)] basic structural units. The activation energies for all studied samples were observed to range from 0.02 to 0.08 eV, and the dielectric constant and the conductivities of the investigated glasses increased with increasing temperature. The electrical behavior of the prepared samples revealed their semiconducting character. The prepared samples are being investigated as potential semiconductor material candidates for use in electronic devices.
Similar content being viewed by others
1 Introduction
Borate glasses are among the most well-known and unique glassy systems. They have useful optical, thermal, and electrical uses and have recently been used as potential bioactive candidates [1]. Recently, borate glasses containing heavy metal oxides as modifiers, such as Bi2O3, were created for a variety of uses, including radiation shielding materials and solid-state lightning uses. Due to the weak field strength of the Bi3+ ions, of all the heavy metal oxides, the existence of bismuth trioxide makes the creation of glass more difficult.
Moreover, both Bi2O3 and B2O3 function well together as network formers and modifiers, enhancing the IR optical transparency, density, moisture resistance, crystallization rate (that decreases), and refractive index (that increases) of the glass materials. Furthermore, Bi2O3 also contains two potential coordination, including pyramidal [BiO3] and octahedral [BiO6] units, which explains how these oxides can function both as glass formers and modifiers in glasses [2, 3].
Bismuth oxide in combination with borate glasses can be used for a variety of purposes, including scintillation detectors in high-energy physics, thermal and mechanical sensors, and wave guides in nonlinear optics. Further, higher luminescence-efficient borate glasses can be created by lowering the phonon energies of the glasses [4]. Transition metal ions are also often employed to study the glass structure, because their outer d-electron orbital functions have a large radial distribution and because of their high sensitivity to environmental changes. Additionally, they are a large range of materials with a variety of interesting features, including magneto resistance, superconductivity, solid-state lasers, piezoelectricity, fiber optic communication, and devices’ luminescent solar energy concentrators [5].
The significant nonlinear optical absorption and electrical characteristics of WO3-containing glasses for technological applications have received a lot of attention [6, 7].
Tungsten ions participate in the glass network with different structural units by two different oxidation states; W6+ as WO4 or W5+ as WO3. W5+ participates in the crystal in the form of W5+O3 which have octahedral positions in the glass structure [8, 9]. Tungsten oxide-based glasses have become increasingly significant in terms of technology over the years despite showing electro- and photo-chromes’ phenomena [10]. In addition, the special features of tungsten trioxide, such as the field strength and high valence of tungsten (VI), promote the formation of networks and improve mechanical properties [11]. Because of its ability to form large areas of glass and change its oxidation from three to four, B2O3 is a very suitable host for the insertion of metal ions. Moreover, the size of the ions in the glass network, the movement of the modifier cation, and other factors have a significant impact on the degree and capability of including WO3 in glasses [12].
Borate glass's refractive index, density, and low phonon energy are improved by adding Bi2O3, MoO3, WO3, and Ga2O3. Additionally, the glass stability is increased by adding glass modifiers made of light elements to borate glass (such as Li+, Na+, Mg2+, etc.). A promising material in the fields of optical and radiation shielding has been thought to be borate glasses combined with light elements and heavy metal oxides. Also, Tungsten oxide has a variety of applications, including the manufacture of 'smart windows,' anti-glare rear view mirrors for cars, non-emissive displays, optical recording systems, solid-state gas, humidity, and temperature sensors [13].
The effect of WO3 in the glassy borate matrix and the dc electrical conductivity of (100–x) Na2B4O7–xWO3 glasses were studied by Al-Shahrani et al. [14]. The authors discovered that an increase in tungsten cation content reduces the ionic conductivity due to an increase in bridging oxygen. Deal et al. [15] reported Raman and luminescence studies on alkali borate tungstate glasses and found that the tetrahedral structural unit WO4 was generated at low WO3 content and increased as the tungsten trioxide content rises. At high content, WO4 and a novel octahedral unite (WO6) were both created. Ali Abou Shama [16] performed structural analyses in Li2O/WO3/B2O3 glasses and also compared them to the other physical parameters that were measured (e.g., activation energy, electrical conductivity, etc.). Alkali tungsten borate glass variations in optical basicity, density, molar volume, and complex impedance were measured by Sheoran et al. [17]. Also, Salem et al. [18] showed physical, structural, optical, and dielectric properties of Li2O/Bi2O3/GeO2/WO3 glasses. Gaffar et al. [19] investigated the FTIR and ultrasonic properties of Li2O/WO3/B2O3 glasses The mixed alkali effect was demonstrated in a recent study of density, glass transition temperature, and electrical characteristics in the Li2O/Na2O/WO3 (MoO3)/P2O5 glass system [17, 20]. More recently, Mahsa et al. [21] studied structural, optical, switching, and antibacterial characteristics of P2O5/B2O3/CuO/Li2O glasses, and these results reveal the specified glass composition's great potential as a window layer [21].
On the basis of these characteristics, heavy metal oxide-based glasses xWO3-(30-x) Bi2O3–15 Li2O–15CaO–40 B2O3 (0 ≤ x ≤ 10 mol %) was produced. Differential thermal analysis, thermal expansion coefficient, electron spin resonance, Raman spectra, and electrical conductivity were thoroughly examined to obtain glasses that can be used in electronic devices.
2 Materials and methods
2.1 Preparation of glass samples
The glasses in the compositions xWO3-(30-x) Bi2O3–15Li2O–15CaO–40B2O3, (x = from 0 to 10 mol %) were prepared as shown before [22]. The chemical compositions of the studied glasses as follows:
-
W0:
15Li2O–15CaO–40B2O3–30Bi2O3–0WO3
-
W1:
15Li2O–15CaO–40 B2O3–27.5 Bi2O3–2.5WO3:
-
W2:
15Li2O–15CaO–40 B2O3–25 Bi2O3–5WO3
-
W3:
15Li2O–15CaO–40 B2O3–22.5 Bi2O3–7.5WO3
-
W4:
15Li2O–15CaO–40 B2O3–20 Bi2O3–10WO3.
2.2 Characterization tools
DTA were measured for finely powdered samples using SEATRAM Instrumentation Regulation, Labsys TM TG-DSC16 (Setaram, Caluire, France) under inert for all studied samples to obtain the glass transition (\({T}_{\mathrm{g}}\)), crystallization (\({T}_{\mathrm{C}}\)), and melting (\({T}_{\mathrm{m}}\)) temperatures.
For thermal expansion, a recording dilatometer (type NETZCH-1-402 PC Germany) was used to obtain the thermal expansion properties with a heating rate of 10 °C/ min up to the softening temperature.
The ESR measurements were carried out using a traditional X-band spectrometer, the Bruker-EMX (Bruker Instruments, Billerica, MA), which has a conventional rectangular cavity (model ER 4102 ST). The spectra were acquired using microwave power of 10 mW at a frequency of 9.77 GHz and a field modulation of 100 kHz with peak-to-peak amplitude of 8 G.
Raman analysis was carried out by a Raman (Ram4.11_1, model; BTC665N-532S-SYS) spectroscopy from 200 to 3500 cm−1.
Using a high-resolution alpha analyzer (0.1–20 MHz) from Novocontrol Technologies, GmbH& Co. KG, the conductivity of the prepared samples was measured in the temperature range of 25–200, and it was stabilized with a precision of more than 0.1 °C using Quattro temperature controllers using pure nitrogen gas as the heating agent. Using typical materials (air, trolitul, and glass) of varying thicknesses, from 1 to 7 mm at 10 kHz, an LCR meter was utilized to calibrate the cell in question. Two Teflon samples of varying thicknesses were used to evaluate calibration curves, and it was discovered that the error in ε´ is ± 2% and the standard deviation are 0.04.
3 Results and discussion
3.1 Thermal analysis
Using the differential scanning calorimetry method, the thermal behavior of glasses was investigated. The values of \({T}_{\mathrm{g}}\) and \({T}_{\mathrm{c}}\) are calculated for each of the examined glasses' DTA analyses, as seen in Fig. 1, and presented in Table 1. The glass transition temperature (\({T}_{\mathrm{g}}\)) reveals data about both the connectivity of the glass network and the strength of interatomic linkages. Glasses with a loosely packed structure have lower \({T}_{\mathrm{g}}\), whereas those with a higher \({T}_{g}\) correlate to a more linked structure [23, 24]. The glass transition temperature (\({T}_{\mathrm{g}}\)) and the exothermic effects associated with the crystallization temperatures of the glasses (\({T}_{\mathrm{c}}\)) are the two characteristics that give all glasses their distinctive hump. With the addition of \({\mathrm{WO}}_{3}\), they develop in the prepared glasses. The creation of new, strong bonds involving tungsten (W–O–Bi; W–O–B) that provide a high degree of connectivity between different structural units and counteract the growth in the amount of non-bridging oxygens is one explanation for the growing \({T}_{\mathrm{g}}\) values that have been observed. Additionally, the bonds that make up these new mixed cross-links have high single-bond dissociation energies (W–O), which could make them stronger and raise \({T}_{\mathrm{g}}\) values [25]. Calculating the relationship between the ideal composition and the stability of glasses cannot be done in a simple manner. The stability of glassy alloys has been assessed quantitatively using a number of different methods.
The majority of them, including Dietzel's technique [26], introduced the first glass parameter \(\Delta\)T = (\({T}_{\mathrm{c}}\)–\({T}_{\mathrm{g}}\)), which is an important measure of glass-forming capabilities. The thermal stability parameter \(\Delta\) T is a crude indicator of the thermal stability of the glass; hence, greater differences between Tc and \({T}_{\mathrm{g}}\) denote more stable glasses [27]. Other parameters were obtained by Saad and Poulain [28]: weighted thermal stability Hg = \(\Delta\)T/\({T}_{\mathrm{g}}\). Greater thermal stability of the glass is reflected by higher results for this parameter. It was discovered that as the \({\mathrm{WO}}_{3}\) concentration increases, the \(\Delta\)T, and Hg values increase as well, enhancing the glass's thermal stability.
3.2 Thermal expansion
The thermal expansion of glasses is influenced by the asymmetry in the amplitude of thermal vibrations inside the glass. As the glass network rigidity increases, it gradually decreases. On the other hand, a change in the coordination number of the network former cation may result in an increase or decrease in the coefficient of thermal expansion depending on how it influences the glass structure [29]. The structure becomes weaker as there are more non-bridging bonds, which in turn results in an increase in the coefficient of thermal expansion. As the WO3 content rises in this investigation, the coefficient of thermal expansion progressively decreases, as seen in Fig. 2. In the temperature range of 25–300 °C, the examined glasses exhibit thermal expansion coefficient values ranging from 12.77 × 10−7 to 9.04 × 10−7 \(^\circ{\rm C}\)−1. The addition of WO3 to the glass network provides a decrease in thermal expansion, because the function units of borate glass convert from BO3 to BO4, which causes the matrix to be rigid [30].
3.3 Electron spin resonance spectroscopy (ESR)
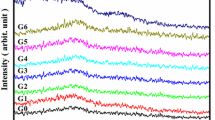
The site symmetry around transition metal ions in glasses is determined using the ESR spectroscopy technique, which also provides detailed information on dynamic and structural features of the local environment of paramagnetic probes in glasses [31].
The ESR spectra of base glass before addition of tungsten ions display no distinct peaks, while those after doping show multi-plate peaks with variable intensities at various applied magnetic fields with different tungsten oxide content, as shown in Fig. 3. Because they have unpaired electrons and so exhibit ESR signals, some tungsten ions are thought to be reduced to lower valence tungsten ions upon addition of tungsten oxide as evidenced by their distinctive optical peaks. The signals are not sharp, because it appears that higher valence tungsten ions, such as the pentavalent and tetravalent states, are also involved [32]. The strengthening and broadening of the ESR signals may also be ascribed to the electron-trapped center at the site of oxygen vacancies. In addition, the increased ESR signals confirm the formation of oxygen vacancy in the studied samples. Thus, rational and controllable doping and vacancy modulation remain challenging task, aimed to the high photocatalytic performance.
3.4 Raman spectral analysis
One method for examining the structure of glass is Raman spectroscopy. The Raman spectra of the prepared glass system in the spectral range of 100–4000 cm–1 are shown in Fig. 4, and they consist of large peaks and shoulders. All spectra have a band at 134 cm–1 that is caused by translational vibrations of the ions \({\mathrm{Bi}}^{3+}\) and \({\mathrm{W}}^{6+}\) ions [33]. Due to the bending vibrations of W–O–W in the WO6 units, which increase in intensity and become narrower with increasing \({\mathrm{WO}}_{ 3}\) content, a broad band with a center at 356 cm–1 is formed [34]. Also, this band may be assigned to Bi–O–Bi vibrations of both [BiO6] and [BiO3] octahedral groups [35]. The stretching vibrations of W–O–W in the WO6 or WO4 groups [36, 37] and/or the stretching vibrations of [BO4] units [38] are attributed to the Raman band approximately 889 cm–1. The weak band at 1228 cm–1 is Bi–O– due to stretching vibrations of [BiO3] triangles [39].
The B–O–-stretching vibrations in various borate group configurations cause the bands with respective centers at 1335 \({\mathrm{cm}}^{-1}\) and 1516 cm–1 [40, 41]. The fact that these bands' intensities decreased in the high wave number region between glass codes W0 and W4 suggests that the tungsten ion contributed to a reduction in the amount of NBO-containing \({\mathrm{BO}}_{ 3}\) unites.
When \({\mathrm{WO}}_{ 3}\) content rises, the separated \({\mathrm{WO}}_{ 4}\) and/or \({\mathrm{WO}}_{ 6}\) units that existed, at lower \({\mathrm{WO}}_{ 3}\) content would gradually merge together, shifting the bands to higher frequencies in the Raman spectra [42]. The quantity of bridging links between tungstate units affects how the bands shift [42, 43]. Between tungstate units, the following bridge bonds can be created: (i) pairs of sharing \({\mathrm{WO}}_{ 4}\) tetrahedra (dimeric \({\mathrm{O}}_{3}\) W–O\(-{\mathrm{WO}}_{ 3}\)); ii) chains of sharing \({\mathrm{WO}}_{ 4}\) & \({\mathrm{WO}}_{ 6}\); (iii) structures of sharing \({\mathrm{WO}}_{ 6}\). If there are only \({\mathrm{WO}}_{ 4}\) dimmers and the amount of \({\mathrm{WO}}_{ 3}\) increases, the number of dimmers may rise, but band shifting should not be seen. The shifting of the bands in connected \({\mathrm{WO}}_{ 6}\) depends on the quantity of W–O–W bridges present in each octahedron [42]. As a result, the frequency increase seen in our spectra can be attributed to the \({\mathrm{WO}}_{6}\) entities' gradual polymerization with increasing \({\mathrm{WO}}_{ 3}\) content. In addition, the generation of another mixed Bi–O–W bonds results in more polarized oxygen atoms than those in Bi–O–Bi and Bi–O–B due to the higher field intensity of W6+ ions (1.47) [44, 45], compared to the field intensity of Bi3+ ions (0.23) [46].
As a result, the oxygen ions are packed more tightly in the glass network. To fill interstitial areas, the increase in \({\mathrm{WO}}_{3}\) content alters the glass network [47].
3.5 Electrical properties
In particular for materials that are employed in electronic devices and insulators, studying the electrical conductivity and dielectric constant of glass materials is essential. Moreover, the effect of changing the frequency and temperature on the ac conductivity and dielectric constants are very important.
As a result, Fig. 5a–e depicts the relationship between frequency–temperature and the logarithm of ac electrical conductivity (σac) for glass samples with various WO3 compositions. It was noted that the ac conductivity increases with increasing the frequency, while at low frequency and an independent frequency conductivity region is observed especially at high temperature values. Jumping of ions forward and backward results in the dispersion of the conductivity values as the frequency increases [48]. In addition, the ac conductivity increases as the temperature rises which indicates the semiconductor nature of the studied samples [49, 50]. The relation between the ac electrical conductivity (σac) and frequency can be described by the universal power law for the samples with [WO3] concentration > 2.5 as the following equation [51]:
where \(\omega\) is the angular frequency, σdc is the dc conductivity, A is a constant which depends on the temperature, and s is the frequency exponent factor (0 < s ≤ 1), which helps in proposing the mechanism of conduction and the extent by which the charge carriers to interact with the environment [48]. However, for the samples with [WO3] concentration < 2.5, the relation between the ac electrical conductivity (σac) and frequency can be described bya double power law [52, 53] given as
The frequency exponent s1 characterized the low frequencies region and s2 representing the high-frequency region. The s values are listed in Table 2 after being calculated from the slopes of log σac versus log f at various temperatures. It was observed that when temperature increases, the frequency exponent decreases, indicating a significant conduction hopping mechanism [54]. The presence of more than one oxidation state for W ions encourages the conduction by transfer of electrons from low to high valance and the charge transfer is considered as small polaron hopping.
As shown in Fig. 5f, W3 sample represents the highest conductivity value at room temperature which may attribute to the creation of non-bridging oxygens as the WO3 content increases where WO3 may act as modifier with WO6 and W5+O3 structural units [55] and as observed in some studies that the increase in the content of WO3 in glass samples can break the tetrahedral linkages of the host glass matrix [8, 9]. The conductivity values of W4 was found to be lower than W3 indicates the importance of the presence of Bi2O3 for the conductivity of the glass samples, where in doped bismuth borate glasses, Bi2O3 contributes to the transformation of tetrahedral units into trigonal units, which creates non-bridging oxygens that lowers the network connectivity and increases the pathway of the carriers (ions) which in turn increasing the conductivity [56].
The variation of the conductivity with the inverse of temperature for samples W0–W4 at low frequency (100 Hz) is shown in Fig. 6. The relation between the conductivity and the temperature is linearly dependent where the conductivity increases with increasing the temperature that may propose the conduction by hopping of charge carrier. From the slope of the linear relation between the conductivity and the temperature, according to the equation, the activation energy \(\Delta E\) is estimated and shown in Table 2 [57]
As shown in Table 3, the lowest value of \(\Delta \mathrm{E}\) is for W3 and the highest is for W4 indicating the decrease in \(\Delta E\) with increasing WO3 and decreasing Bi2O3 content. The increase in conductivity and the decrease in \(\Delta E\) with increasing WO3 content to certain value and then the behavior reversed are in good agreement with the previous findings [13]. The values of \(\Delta E\) are very low when compared to other borate and phosphate glasses [13, 50, 58] due to the presence of Bi2O3 (act as a network modifier) which increases the conversion of units [BO4] to [BO3] that leading to the creation of non-bridging oxygens [29, 56]. Low values of activation energy indicate that the transport occurred by electron tunneling [59].
The Nyquist diagrams which represent the relation between the real and imaginary parts of the ac-impedance of the samples (W0–W4) at room temperature are shown in Fig. 7a). A single depressed semi-circle correlated to a parallel combination of both the capacitance and resistance of the bulk. The radius of the semi-circle decreases with increasing the WO3 content indicating the increase in the bulk sample conductivity till W3, and then, it increases for W4 to be larger than W3 but still lower than all the samples. This behavior is coincidence with the conductivity measurements and indicates the importance of presence of least amount of Bi2O3 in the bulk conductivity of the samples.
The Nyquist plots of W3 sample at various temperatures are displayed in Fig. 7b. The radius of the semi-circle decreases as the temperature increase, indicating that the conduction is thermally dependent.
Dielectric constant dependence on both frequency and temperature for W0–W4 is shown in Fig. 8a–e. High values of dielectric constant were observed at low frequency which explained by the polarization of the sample. Definitely, in amorphous glasses, the high dielectric constant at lower frequencies may be due to the defect in the bulk interfacial. Therefore, when the space charges move under the applied field, they may be surrounded by the defects and many space charge polarization occurred [60]. A gradual decrease in the dielectric constant (ε′) for all samples was detected with increasing the frequency and ε′ seemed to be independent on frequency at the high-frequency range due to the disability of the charge carriers to sufficiently rotate, but they oscillate to place behind the field that results in the decrease in ε′ [61, 62].
The dielectric constant (έ) for the samples W0–W4 increases with rising the temperature where the temperature causes vibration of the molecules leading to the orientation polarization [63]. The increase έ with temperature results also from the presence of bonding defects that causes space charge polarization [64]. The values of dielectric constant for the samples of low WO3 content (W0–W2) at low frequencies are low ranging from 5 to 80 indicating semiconductor properties, while for W3& W4, the έ values are high reaching 1800.
Tangent loss factor (\(\mathrm{tan\theta }\)) is used to calculate the phase difference caused by the sample's loss of energy, and therefore, tangent loss for sample W3 was calculated according to the relation
where \(\varepsilon^{\prime\prime}\) and \(\varepsilon^{\prime}\) are the imaginary and real parts of the permittivity. The temperature-frequency dependence of tangent loss for W3 sample is shown in Fig. 8f. It is noted that the tangent loss at low frequencies (100 Hz and 1 kHz) increases with increasing the temperature; however, at high frequencies (10 kHz and 100 kHz), it decreases with temperature till 373 K (100 °C) and then increases with the rise of temperature.
The dielectric response could be tracked also by studying the variation of the real part of electric modulus M', and its imaginary part M'' for W3 sample with the frequency at different temperatures as presented in Fig. 9a, b. The electric modulus is used to understand the bulk response of the conducting materials and reducing the polarization effect [65, 66]. The values of M' at low frequencies are small and increase to reach a maximum value at high-frequency values demonstrating the spreading of the relaxation processes over the whole range of frequencies [66]. The relation of M'' with frequency and temperature (Fig. 9b) shows one asymmetric peak at low-frequency temperature shifted to higher frequency and lower peak height with increasing the temperature, while there is an indication of another peak at high-frequency values where its height decreases with increasing the temperature (inset Fig. 9b). The frequency at which the peak appeared is known as the relaxation frequency (fM''), and at frequencies more than fM'', the charge carriers are restrained and are moved over short distance [67].
4 Conclusion
The purpose of this study was to identify whether increasing the WO3 content of glasses would improve their DTA, TEC, ESR, Raman spectra, and electrical properties. Hence, we produced numerous sample types with various WO3 contents (0 ≤ x ≤ 10 mol %). The samples were produced using meltquenching method. Both \({T}_{\mathrm{g}}\) (from 423°C to 440°C) and \({T}_{c}\) (from 478°C to 543°C) increase with the addition of \({\mathrm{WO}}_{3}\) into the prepared glasses due to the creation of new strong bonds with participation of tungsten. The coefficient of thermal expansion gradually decreases from and Tc (from 478°C to 543°C) increase with the addition of WO3 into the prepared glasses due to the creation of new strong bonds with participation of tungsten. The coefficient of thermal expansion gradually decreases from 12.77 × 10−7/°C to 9.04 × 10−7/°C with increasing WO3 content. ESR demonstrates the presence of W5+ ions, which have unpaired electrons, exhibit distinct ESR signals that reveal high intensities as WO3 content rises. Raman spectra analyses supported the presence of the structural groups [\({\mathrm{BiO}}_{6}\)], [\({\mathrm{BO}}_{3}\)], [\({\mathrm{BO}}_{4}\)], and [\({\mathrm{WO}}_{4}\)] in the glasses. The dielectric constant and the conductivities of the studied glasses increased with increasing temperature revealing semiconducting behaviour for the samples, and a large hopping mechanism of the conduction was suggested. From these results, it can be concluded that the investigated glasses can be used in electronic devices.
References
J.E. Shelby, Introduction to Glass Science and Technology (Royal Society of Chemistry, Cambridge, 2005)
R.N.A. Prasad, B.V. Siva, K. Neeraj, N. Krishna Mohan, J.I. Rojas, Influence of modifier oxides on spectroscopic features of Nd2O3 doped PbO-Ro2O3–WO3–B2O3 glasses (with Ro2O3 = Sb2O3, Al2O3, and Bi2O3). J. Lumin. 223, 117171 (2020). https://doi.org/10.1016/j.jlumin.2020.117171
E.K. Abdel-Khalek, E.A. Mohamed, S.M. Salem, I. Kashif, Structural and dielectric properties of (100–x)B2O3-(x/2)Bi2O3–(x/2)Fe2O3 glasses and glass-ceramic containing BiFeO3 phase. J. Non-Cryst. Solids 492, 41–493 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.04.020
G. Lakshminarayana, K.M. Kaky, S.O. Baki, A. Lira, A.N. Meza-Rocha, C. Falcony, U. Caldiño, I.V. Kityk, A. Mendez-Blas, A.F. Abas, M.T. Alresheedi, M.A. Mahdi, Nd3+-doped heavy metal oxide based multicomponent borate glasses for 1.06 μm solid-state NIR laser and O-band optical amplification applications. Opt. Mater. 78, 142–159 (2018). https://doi.org/10.1016/j.optmat.2018.02.011
N.J. Kreidl, Recent applications of glass science. J. Non-Cryst. Solids 123(1–3), 377–384 (1990)
K.S. Rao, V.R. Kumar, N. Veeraiah, Dielectric, magnetic and spectroscopic properties of Li2O –WO3–P2O5 glass system with Ag2O as additive. Mater. Chem. Phys. 111(2–3), 283–292 (2008)
S.M. Salem, Effect of iron on the electrical properties of lead–bismuth glasses. J. Mater. Sci. 44, 5760–5767 (2009)
G. Raju, R.B. Rao, P.N. Rao, N. Veeraiah, in Focus on Condensed Matter Physics Research, Chap. 5. ed. by J.V. Chang (Nova Science Publisher Inc, New York, 2005), p.147
S. Dirke, S. Müller, K. Bärner, H. Rager, Cluster formation of WO3 in Li2B4O7 glasses. J. Non-Cryst. Solids 124, 265–270 (1990)
G. Upender, C. Sameera Devi, V. Chandra Mouli, Role of WO3 on DC conductivity and some optical properties of TeO2 based glasses. Mater. Res. Bull. 47, 3764–3769 (2012)
J. Han, F. He, L.L. Wang, L.X. Zhang, C.Q. Ye, J.L. Xie, S.X. Mei, M.F. Jin, Effect of WO3 on the structure and properties of low sintering temperature and high strength vitrified bonds. J. Alloys Compd. 679, 54–58 (2016)
R. Iordanova, M. Milanova, L. Aleksandrov, A. Khanna, Structural study of glasses in the system B2O3-Bi2O3-La2O3-WO3. J. Non-Cryst. Solids 481(1), 254–259 (2018)
G.P. Singh, P. Kaur, S. Kaur, D.P. Singh, Role of WO3 in structural and optical properties of WO3–Al2O3–PbO–B2O3 glasses. Phys. B 406, 4652–4656 (2011)
A. Al-Shahrani, A. Al-Hajry, M.M. El-Desoky, Non-adiabatic small polaron hopping conduction in sodium borate tungstate glasses Phys. Stat. Sol. (a) 200(2), 378–387 (2003). https://doi.org/10.1002/pssa.200306689
D. Deal, M. Burd, R. Braunstein, Raman and luminescence studies of alkali borate tungstate glasses. J. Non-Cryst. Solids 54(3), 207–221 (1983). https://doi.org/10.1016/0022-3093(83)90066-2
A.A. Shama, Structural investigation of amorphous xLi2O. xWO3. (1–2x)B2O3 correlated with its physical properties. Egypt. J. Solids 31(1), 63–74 (2008)
A. Sheoran, S. Sanghia, S. Rani, A. Agarwal, V.P. Seth, Impedance spectroscopy and dielectric relaxation in alkali tungsten borate glasses. J. Alloys Compd. 475, 804–809 (2009)
S.M. Salem, E.K. Abdel-Khalek, E.A. Mohamed, M. Farouk, A study on the optical, structural, electrical conductivity and dielectric properties of a lithium bismuth germanium tungsten glasses. J. Alloys Compd. 513, 35–43 (2012)
M.S. Gaafar, Y.B. Saddeek, L. Abd El-Latif, Ultrasonic studies on alkali borate tungstate glasses. J. Phys. Chem. Solids 70(1), 173–179 (2009)
S. Suresh, M. Prasad, V. Chandra mouli, Ac conductivity and impedance measurements in alkali boro tellurite glasses. J. Non-Cryst. Solids 356(31–32), 1599–1603 (2010)
M. Baazm, E. Soheylia, M.H. Hekmatshoar, A. Rostamzad, A.K.C. Abad, Preparation of quaternary boro-phosphate multifunctional glasses and their structural, optical, switching and antibacterial properties. Ceram. Int. 44(8), 9414–9421 (2018)
A.A. Ali, S. Ibrahim, E.M. Ahmed, Y.S. Rammah, Influence of WO3 on gamma radiation shielding efficiency, physical and optical properties of newly developed Li2O – CaO – Bi2O3 – B2O3 glasses. Radiat. Phys. Chem. 198, 110240 (2022)
Y.B. Saddeek, M.A. Azooz, A.B. Saddek, Ultrasonic investigations of some bismuth borate glasses doped with Al2O3. Bull. Mater. Sci. 38(1), 241–246 (2015)
N.H. Ray, Composition-property relationship in inorganic oxide glasses. J. Non-Cryst. Solids 15, 423–434 (1974)
M. Milanova, K.L. Kostov, R. Iordanova, L. Aleksandrov, A. Yordanova, T. Mineva, Local structure, connectivity and physical properties of glasses in the B2O3-Bi2O3-La2O3-WO3 system. J. Non-Cryst. Solids 516, 35–44 (2019)
M. Saad, M. Poulain, Glass forming ability criterion. J Mater Sci Forum 19–20, 11–18 (1987). https://doi.org/10.4028/www.scientific.net/MSF.19-20.11
J. Sestak, Heat as manufacturing power or the source of disorder. J. Therm. Anal. 69(1), 113–124 (2002). https://doi.org/10.1023/a:1019989707529
H.S. Chen, Alloying effect on the viscous flow in metallic glasses. J. Non-Cryst. Solids 27, 257–263 (1978)
S.P. Singh, B. Karmakar, Synthesis and characterization of low softening point high Bi2O3 glasses in the K2O–B2O3–Bi2O3 system. Mater. Charact. 62, 626–634 (2011)
S. Stalin, D.K. Gaikwad, M.S. Al-Buriahi, C. Srinivasu, S.A. Ahmed, H.O. Tekin, S. Rahman, Influence of Bi2O3/WO3 substitution on the optical, mechanical, chemical durability and gamma ray shielding properties of lithium-borate glasses. Ceram. Int. 47(4), 5286–5299 (2021)
A. Abragam, B. Bleaney, Electron Paramagnetic Resonance of Transition Ions (Clarendon Press, Oxford, 1970)
R. Staske, P. Frobel, K. Barner, Fluorescence of Sm3+ in Sm/Cu-codoped (Li2B4O7)1−x(WO3)x glasses. J. Lumin. 55(3), 115–123 (1993)
M. Mączka, M. Ptak, L. Kępinski, P.E. Tomaszewski, J. Hanuza, X-ray, SEM, Raman and IR studies of Bi2W2O9 prepared by Pechini method. Vib. Spectrosc. 53, 199–203 (2010)
M. Mączka, V. Nikolov, K. Hermanowicz, A. Yordanova, M. Kurnatowska, J. Hanuza, Optical and phonon properties of nanocrystalline Al2(WO4)3 doped with chromium(III) prepared by co-precipitation method. Opt. Mater. 34, 1048–1053 (2012)
B. Shashidhar, S. Rahman, A.M. Awasthi, V. Sathe, Role of Bi2O3 content on physical, optical and vibrational studies in Bi2O3–ZnO–B2O3 glasses. J. Alloys Compd. 460, 699–703 (2008)
G. Poirier, Y. Messaddeq, S.J.L. Ribeiro, M. Poulain, Structural study of tungstate fluorophosphates glasses by Raman and X-ray absorption spectroscopy. J. Solid State Chem. 178, 1533–1538 (2005)
E.I. Ross-Medgaarden, I.E. Wachs, Structural determination of bulk and surfacetungsten oxides with UV−vis diffuse reflectance spectroscopy and Raman spectroscopy. J. Phys. Chem. C 11, 15089–15099 (2007)
S. Bale, M. Purnima, Ch. Srinivasu, S. Rahman, Vibrational spectra and structure of bismuth based quaternary glasses. J. Alloys Compd. 457, 545–548 (2008)
F. He, J. Wang, D. Deng, Effect of Bi2O3 on structure and wetting studies of Bi2O3–ZnO–B2O3 glasses. J. Alloys Compd. 509, 6332–6336 (2011)
L. Baia, R. Stefan, J. Popp, S. Simon, W. Kiefer, Vibrational spectroscopy of highly iron doped B2O3–Bi2O3 glass systems. J. Non-Cryst. Solids 324, 109–117 (2003)
D. Maniu, T. Iliescu, I. Ardelean, S. Cinta-Pinzaru, N. Tarcea, W. Kiefer, Raman study on B2O3–CaO glasses. J. Mol. Struct. 651, 485–488 (2003)
V.I. Tsaryuk, V.F. Zolin, Vibration and vibronic spectra of lanthanide compounds with different types of coordination polyhedra of tungsten and molybdenum. Spectrochim. Acta A 57, 355–359 (2001)
G. Poirier, Y. Messaddeq, S.J.L. Ribeiro, M. Poulain, Structural study of tungstate fluoro phosphate glasses by Raman and X-ray absorption spectroscopy. J. Solid State Chem. 178, 1533–1538 (2005)
D. Minoz-Martin, M.A. Villegas, J. Gonzalo, J.M. Fernandez-Navaro, Characterization of glasses in the TeO2-WO3-PbO system. J. Eur. Ceram. Soc. 29, 2903–2913 (2009)
M. Celikbilek, A. Ercin Ersundu, S. Aydin, Glass formation and characterization studies in the TeO2-WO3-Na2O system. J. Am. Ceram. Soc. 96(5), 1470–1476 (2013)
Q. Chen, Q. Ma, H. Wang, Q. Wang, Y. Hao, Q. Chen, Properties and structure of faraday rotating glasses for magneto optical current transducer. Bol. Soc. Esp. Ceram. Vidrio 56, 1–12 (2017)
S. Stalin, D.K. Gaikwad, M.S. Al-Buriahi, C. Srinivasu, S.A. Ahmed, H.O. Tekin, Syed Rahman, Influence of Bi2O3/WO3 substitution on the optical, mechanical, chemical durability and gamma ray shielding properties of lithium-borate glasses. Ceram. Int. 47, 5286–5299 (2021)
A.A. Ebnalwaled, Hopping conduction and dielectric properties of In Sb bulk crystal. Int J Basic Appl Sci. 11 (2011).
G.B. Devidas, T. Sankarappa, M.P. Kumar, S. Kumar, AC conductivity in rare earth ions doped vanadophosphate glasses. J. Mater. Sci. 43, 4856–4861 (2008)
R.M.M. Morsi, A.M. Abdelghany, M.M. Morsi, Dielectric, electrical and spectroscopic properties of barium borates of low WO3 content. J Mater Sci: Mater Electron 26, 5120–5128 (2015)
S.R. Elliott, A.c. conduction in amorphous chalcogenide and pnictide semiconductors. Adv Phys 36(2), 135–217 (1987). https://doi.org/10.1080/00018738700101971
K. Funke, Jump relaxation in solid electrolytes. Prog. Solid State Chem. 22, 111–195 (1993)
D.P. Almond, C.R. Bowen, Anomalous power law dispersions in ac conductivity and permittivity shown to be characteristic of microstructural electrical networks. Phys. Rev. Lett. 92, 5 (2004)
A.A. Ali, M.H. Shaaban, Electrical properties of LiBBaTe glass doped with Nd2O3. Solid State Sci. 12, 2148–2154 (2010)
P. Frobel, K. Barner, Optical properties of some [[(Li2O) · (B2O3)2]1 − x(WO3)x]1 − yMyo glasses. J. Non-Cryst, Solids 88, 329–344 (1986)
M. Mariyappan, K. Marimuthu, M.I. Sayyed, M.G. Dong, U. Kara, Effect Bi2O3 on the physical, structural and radiation shielding properties of Er3+ ions doped bismuth sodium fluoroborate glasses. J. Non-Cryst. Solids Solids 499, 75–85 (2018)
N. Mott, E.J.C. Davis, Oxford, Electronic processes in non-crystalline semiconductors, 220 (1979)
S.A.M. Abdel-Hameed, A.M. Fathi, M. Eltohamy, Structure, optical and electrical behaviour of x (2Bi2O3,MnO). (10–x)B2O3 glasses. J. Non-Cryst. Solids 510, 71–80 (2019)
S.A.M. Abdulhamid, A.M. Fathi, Preparation and characterization of silver nanoparticles within silicate glass ceramics via modification of ion exchange process. J. Alloys Compd. 498, 71–76 (2010)
A.A. Bahgat, Y.M. Abou-Zeid, Mixed alkali effect in the K2O-Na2O-TeO2, glass system. Phys. Chem. Glasses 42, 361–370 (2001)
P. Rosaiah, O.M. Hussain, Synthesis, electrical and dielectrical properties of lithium iron oxide. Adv. Mat. Lett. 4(4), 288–295 (2013)
M.A. Ramadan, A.M. Fathi, S. Sharawy, M.K. Elbisi, A new approach for preparation of smart conductive textiles by polyaniline through in-situ polymerization technique. Egypt. J. Chem. 61, 370–380 (2018)
D.E. Day, Mixed alkali glasses—their properties and uses. J. Non-Cryst. Solids 21, 343 (1976)
E. Mansour, Mixed alkali effect in quaternary K2O–Li2O–BaO–B2O3 glasses containing V2O5. J. Phys. B 362, 88 (2005)
A. Śantić, C.W. Kim, D.E. Day, A. Moguś-Milanković, Electrical properties of Cr2O3-Fe2O3-P2O5 glasses. Part II. J. Non-Cryst. Solids 356, 2699–2703 (2010)
S.A.M. Abdel-hameed, A.M. Fathi, R. Elwan, F.H. Margha, Effect of F− and B3+ ions and heat treatment on the enhancement of electrochemical and electrical properties of nanosized LiTi2(PO4)3 glass-ceramic for lithium-ion batteries. J. Alloys Compd. 832, 154943 (2020)
A. Sheoran, S. Sanghi, S. Rani, A. Agarwal, V.P. Seth, Impedance spectroscopy and dielectric relaxation in alkali tungsten borate glasses, J. Alloys Compd. 475, 804–809 (2009)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
AAA: conceptualization, methodology, validation, investigation, data curation, writing—original draft, and writing—review & editing. AMF: conceptualization, methodology, validation, investigation, data curation, writing—original draft, and writing—review & editing. SI: conceptualization, methodology, validation, investigation, data curation, writing—original draft, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.A., Fathi, A.M. & Ibrahim, S. Material characteristics of WO3/Bi2O3 substitution on the thermal, structural, and electrical properties of lithium calcium borate glasses. Appl. Phys. A 129, 299 (2023). https://doi.org/10.1007/s00339-023-06537-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06537-w