Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.”
NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K
Relation: The relation between pressure (P) volume (V), number of moles (n) and temperature (T) remain the same as in other gas laws:
- Pressure (P) is indirectly proportional to volume (V).
- Volume (V) is directly proportional to temperature (T) and number of moles (n).
- Temperature (T) is directly proportional to Pressure (P).
Equation: PV = nRT
NOTE: This gas law must use the same units as those found in the Ideal Gas Constant, R—that means only SI units! Otherwise the units will not cancel out when the equation is solved.
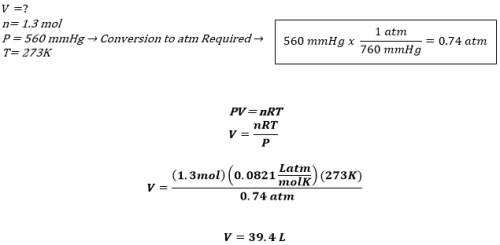
Problem: What is the volume of 1.3 mol of HCl gas at 560mmHg pressure and 273K?
GIVEN: